The Three Day Salvia Trip...?
The horrifying medicinal chemistry of super-potent, irreversible, salvinorin analogues.
Somewhat echoing Albert Hofmann’s unintentional 250 microgram LSD power-dose almost exactly 50 years prior, American ethnobotanist Daniel Siebert’s first attempt at smoking a homemade Salvia divinorum extract on June 6th 1993 would turn out to be a reality-shattering overshoot. Unsure as to the activity of the crystalline material he’d isolated from the plant’s leaves, Siebert “decided to play it safe” and smoked what he estimated would be a conservative dose — 2.5 mg:
“Quite suddenly I found myself in a confused, fast moving state of consciousness with absolutely no idea where my body or my universe had gone.”
Siebert lost all memory of ever having existed as a human being and struggled desperately to find a way back to some kind of recognisable reality:
“I realized that I had no actual memory of ever having existed in any other state of consciousness than the disembodied one I was now in. So I decided to stop panicking and just relax. After all, there was no place to get back to. I was totally convinced that this state of existence was all there ever was.”
Siebert’s trip lasted for another 10-15 minutes, during which he was catapulted between various scenes from his past, including the living room of his long-dead grandparents, before being returned to the normal waking world “shaken to the soul” but elated at having discovered the psychedelic essence of Salvia divinorum, now known as salvinorin A. Subsequent analyses revealed Siebert’s extract to be around 80% pure — a 2mg dose (1 mg is considered very heavy).
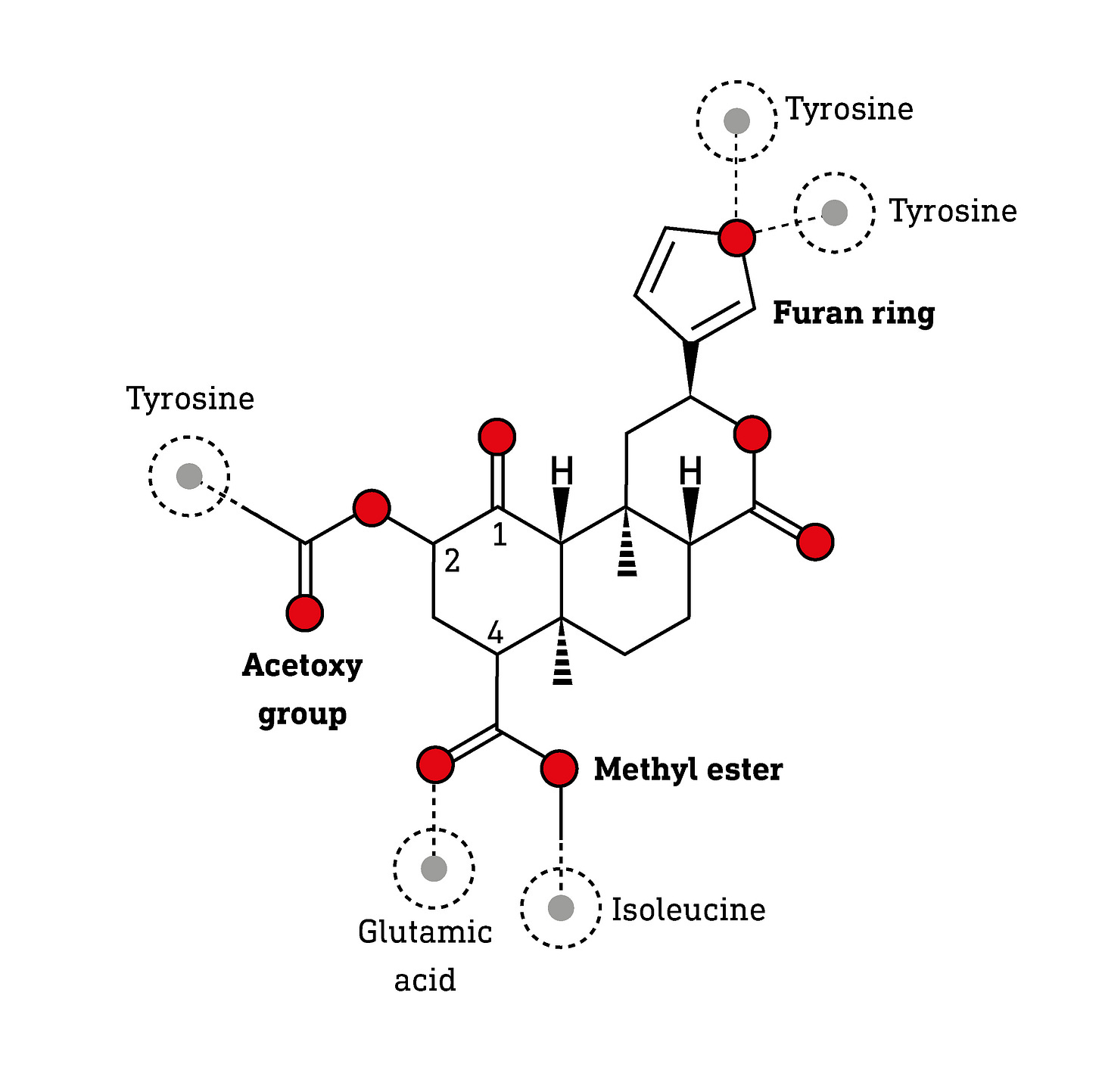
Salvinorin A was an entirely unexpected psychedelic molecule, not only in terms of its structure, but also its mechanism of action in the brain. All the known naturally-occurring psychedelics, prior to the discovery of salvinorin A, were nitrogen-containing alkaloids. Salvinorin A — the first of ten closely-related salvinorins (A-J) to be isolated from the Salvia divinorum plant — belongs to an entirely different class of natural molecules: the terpenes. Whereas the alkaloids are built using amino acids as the starting material, the terpenes are derived by stitching together a simple three-carbon molecule, isoprene, to form chains that are then folded to form complex ringed structures. Salvinorin is a diterpene, a 20-carbon molecule built by joining four isoprene subunits to yield geranylgeranyldiphosphate, which then undergoes a cascade of carbon-carbon bond forming reactions to generate salvinorin’s characteristic three ring skeleton.
The classic psychedelics, such as LSD and psilocin, work by activating 5HT2A receptors embedded in neurons deep in the cerebral cortex. This increases the activity of these neurons and allows entirely novel patterns of activity to emerge, experienced as the psychedelic state. Salvinorin, on the other hand, achieves its effects via a completely different and entirely unique mechanism. Rather than activating the 5HT2A serotonin receptors, salvinorin binds and activates a type of opioid receptor known as the kappa receptor, a member of the family of receptors also responsible for the effects of opiates, such as morphine.
See my previous post for more about salvinorin’s mechanism in the brain:
Although concentrated Salvia extracts experienced a surge in popularity in the 2010s, I rarely meet people who find the trip anything other than traumatic and frankly horrifying. And, unlike with DMT, I’ve never met anyone complaining that the trip is too short — 5-10 mins of pure existentially absolute madness is almost always quite enough, if not way too much. However, owing to their action at the kappa opioid receptor, there has been a lot of interest in adapting the salvinorin molecules for the clinic, by converting them from kappa opioid agonists (responsible for the psychedelic effects) to mu opioid agonists — the site of analgesic activity of the opiates.
Salvinorin’s three ring skeleton is adorned with an unusual furan ring that forms two hydrogen bonds with tyrosine amino acids in the kappa receptor binding site. The 4-carbon contains a methyl ester group that interacts with glutamic acid and isoleucine amino acids and the 2-carbon contains an acetoxy group that forms hydrophobic interactions with another tyrosine (LINK).
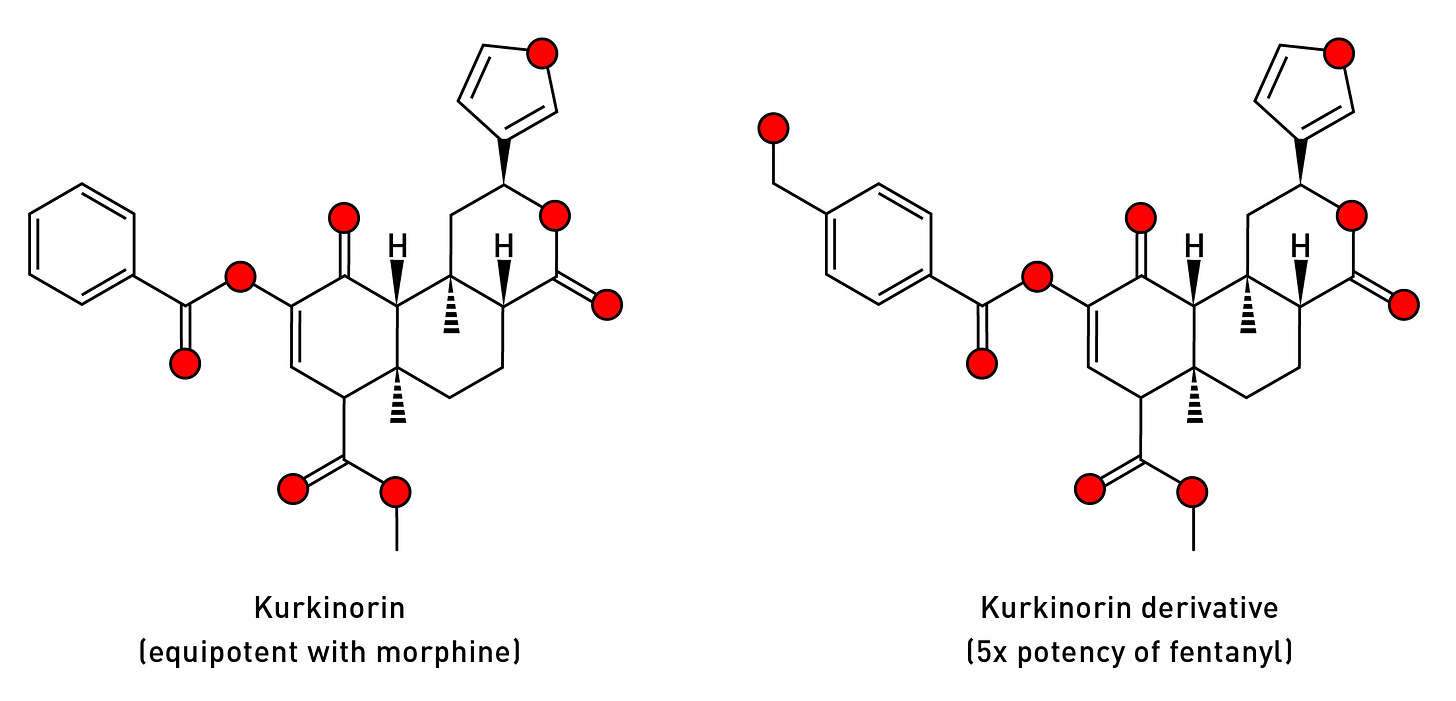
Although there have been some attempts at creating active analogues by attaching groups to the furan ring, almost all changes to this part of the molecule dramatically reduce potency, and most studies have focused on the acetoxy group at the 2-carbon. Kurkinorin, for example, is derived from salvinorin A by adding a phenyl ring to this group (in addition to a double bond in one of the rings). Kurkinorin is equipotent with morphine, but doesn’t exhibit many of the unwanted side-effects, such as sedation, respiratory depression, and the potential for addiction (LINK). A recently-synthesised hydroxylated derivative of kurkinorin is even more effective, being five times more potent than fentanyl at the mu receptor, but with no activity at the kappa subtype (LINK). It’s quite remarkable how relatively small structural changes to a molecule can completely alter its pharmacological properties.
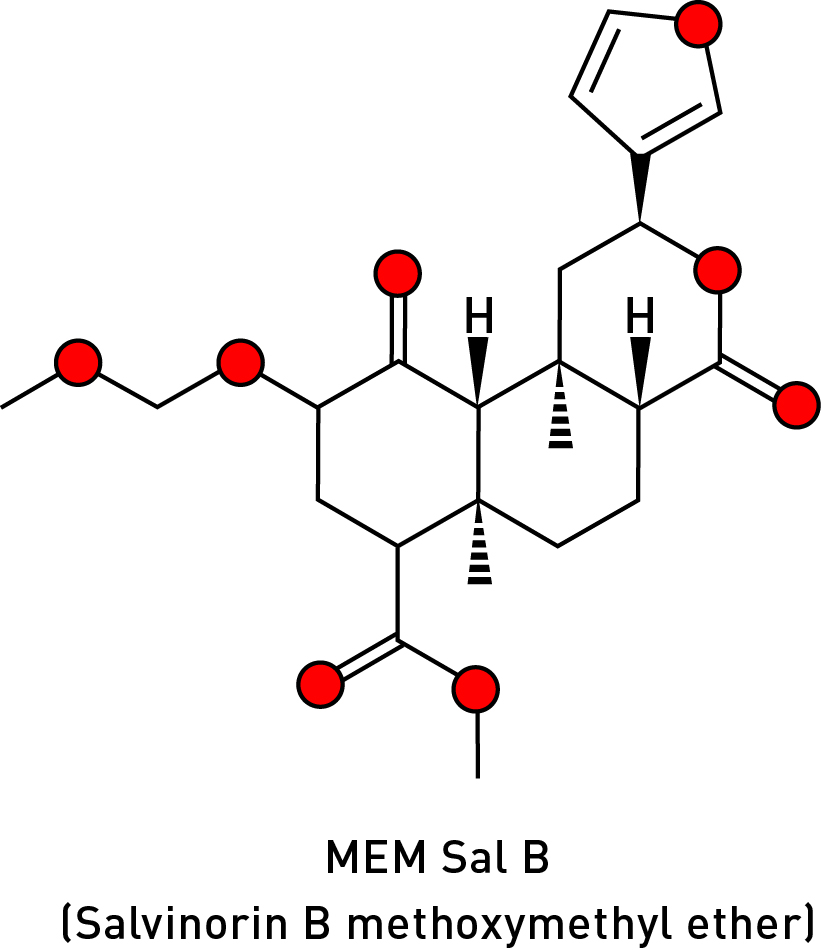
In 2005, Bruce Cohen of Harvard Medical School synthesised a series of salvinorin analogues by replacing the 2-carbon acetoxy with a range of groups and testing their activity against the kappa receptor. Whilst most of these analogues were either much less potent than salvinorin A/B or completely inactive, the methoxymethyl ether analogue (MEM Sal B) was found to be about nine times more potent than the parent compound with a much higher affinity for the kappa receptor (LINK).
Subsequent studies in mice showed that not only does MEM Sal B substitute completely for salvinorin A but, rather than a few minutes, the mice were completely immobilised for three hours! (LINK). It’s hard to imagine why anyone would want to try this molecule on themselves and, perhaps not surprisingly, I was only able to find one trip report — on the Erowid site — which is worth quoting at length:
"I measured out 50ug and smoked it. No effects. Then 100ug. Slight buzz, no effects other than that. I decided to throw in 275ug….I took the hit. I held it. Within five seconds I felt something. My knees started warping and stretched out about twenty feet and went through my door. I stood up, and my head blasted through the ceiling, and before I knew it I was standing above my city. I could see each and every person doing their own thing. I tried to bend down and look further, and reappeared in my house before disappearing and falling through my floor into an endless array of eerie sounds. I eventually reached the bottom and felt my body hit the ground. A creature walked toward me and grabbed me by the neck and said ‘You better know what you’re doing’ in a disturbing voice. Then he threw me and I landed. I stood up and realized that I was in the 1950s…
The trip went from impossible events to me simply existing in a different time. I walked around on this street, and found a newspaper on the ground. I could read each and every article. At the top read, ‘August 6, 1953.’ I didn’t know that it wasn’t real. It was all that I knew. I could remember my life, but it wasn’t mine. It was the life of the person that I was in 1953. I could remember my childhood, school years, first job, everything. I went into a restaurant. I walked to the counter and asked for a hot dog. The woman said no problem and I sat down. A few minutes later, she brought over my order. I began to bite in before I saw that instead of a hot dog between buns it was my real self, wiggling between the bread. I freaked out, but didn’t know who it was. I was wondering what was going on, because I felt like something was wrong in the person that I was during this time. I began to feel lightheaded, and everything went black. I rolled over, and I was back to reality. I had no idea who I was. What anything was. My ceiling was falling apart and breaking into little pieces everywhere. Anything that I looked at had a face and smiled at me…
About 10 minutes later, most of this subsided, and I realized what happened. My mind was blown. This was an infinite amount stronger than my strongest experience on Salvia. I went and looked at my clock. Nearly 3 hours had passed.” (LINK)
Although it’s impossible to verify this single trip report, these kinds of “substitute life” experiences, in which the tripper finds themselves embedded in an entirely unpsychedelic and coherent alternate life, complete with family, friends, and a full life history, have been reported before with concentrated Salvia extracts. The three hour duration also checks out, and the synthesis of MEM Sal B isn’t too difficult for anyone with a modicum of chemistry know-how, so the report seems quite plausible, albeit horrifying.
OK, so a three hour salvinorin trip already seems far beyond anything any sane human would desire to experience. But what about three days?
The effect duration of a psychedelic depends partly on how long it sticks to the receptor — in a previous post I discussed how LSD’s characteristically long trip can be explained by the unique manner in which it binds to the 5HT2A receptor:
The binding of a molecule to a receptor usually depends upon a range of relatively weak, reversible, bonds: ionic bonds, hydrogen bonds, and hydrophobic interactions. However, it’s also possible to create so-called irreversible agonists that form strong covalent bonds with amino acids within the binding site. Unlike standard reversible agonists, once bound, these agonists remain in the binding site permanently (or, at least, until the receptor is internalised for recycling or degradation). Whilst such molecules are rarely of clinical use in humans, they can be excellent tools for studying receptors in the laboratory.
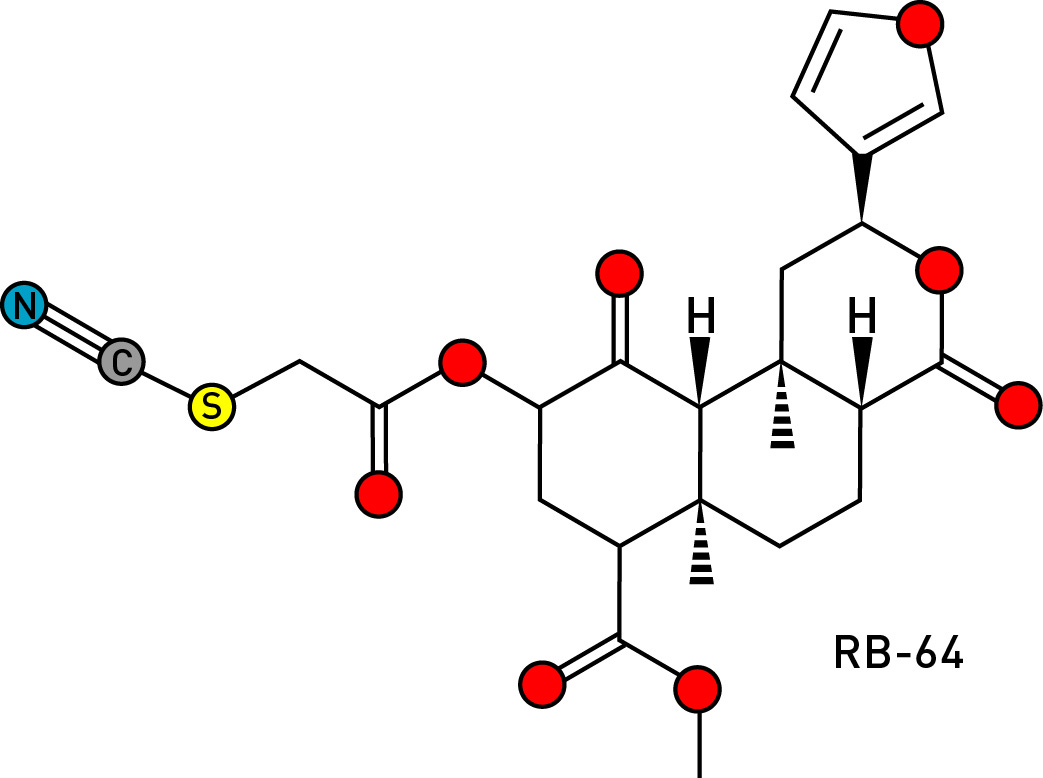
In 2009, Bryan Roth’s group produced a salvinorin analogue by replacing the C2 acetoxy group with a thiocyanate group (LINK). This is a reactive group that can form a covalent bond with a cysteine amino acid suitably positioned in the kappa receptor binding site. Known as RB-64, this irreversible agonist was found to be “extraordinarily potent”: 20x more potent than salvinorin A in standard tests for psychedelic activity in mice.
If this potency translated to humans, this would mean a fully psychedelic dose as low as 10 micrograms — considerably more potent than LSD and with a much longer duration of action. How long? It’s really quite hard to say, since activation of receptors by agonists eventually leads to their internalisation, although this effect seems to be muted with RB-64 compared to salvinorin A. However, a three day trip certainly isn’t out of the question, although I can’t for the life of me imagine anyone wanting to undergo such an ordeal, nor what the psychological consequences might be. This is one molecule that should remain in the laboratory.
It should be pointed out that RB-64 is a biased agonist with more potent effects on G-protein signalling vs beta-arrestin signalling (again, see my recent LSD post for more on biased agonism: LINK). It isn’t yet clear how this signalling balance relates to psychedelic effects at the kappa receptor and thus whether RB-64 would even be psychedelic in humans, although well-established rodent models indicate extremely potent “psychedelic activity”…
So, naturally, there’s only one way to find out…









Very interesting (and horrifying) post, thanks so much. I was wondering if (and if not, why not) any secret services have ever used such compounds to poison enemies or captives (mk ultra aside). Russia has an ongoing poisoning program with radioactive polonium that killed Litvinenko, Skripal and Navalny nearly died of an agent called Novichok. And the Russians for sure arent alone here, thinking about failed American attempts to poison Fidel Castro.
Not expecting a definite answer of course (and I hope I am not giving anyone nee ideas ;-)) but that would make a lot of sense, wouldn't be hard to manufacture for a government or to dose a target...
Sounds like a real good time. Where can I score ?