The principal aim of DMTx is to use a programmable intravenous infusion device to reach and maintain a relatively stable concentration of DMT in the brain such that the standard 5-10 minute breakthrough trip can be extended for an indefinite period of time. Although this idea seems simple in principle, as is so often the case in human pharmacology, it’s actually rather complicated. So, in this post, I want to discuss in more detail the science behind this technology.
I discussed the history and potential applications of DMTx in the last post, so please read that if you haven’t already:
Even those without any training in pharmacology will understand that a drug must first get into the blood and then find its way to the site of action — in the case of DMT, the brain — all the while fighting against the body’s natural response to exogenous molecules, which is to break them down and get rid of them. Pharmacokinetics is the branch of pharmacology that measures and quantifies these processes, such that we can predict — to a greater or lesser extent — how a particular drug will behave after it’s administered.
If you take any courses in pharmacology, one of the first central concepts you’ll learn goes by the initialism ADME: absorption, distribution, metabolism, excretion (sometimes a “T” for toxicity is tacked onto the end). Broadly, ADME refers to what happens to a drug when it enters your body, whether by oral, intramuscular, intravenous, or any other route.
More specifically:
Absorption: How does the drug get into your bloodstream and at what rate? With IV administration this is easy to calculate, since it’s just the rate at which the drug is injected. The blood is referred to as the central compartment.
Distribution: Where does the drug go once it’s in the blood? Obviously, we’re most interested in how it gets into the brain, which is known as the effect compartment. However, depending on its chemical properties, a drug will usually leave the bloodstream and distribute to different degrees in other organs, soft tissues, muscles, and fat. These are together referred to as the peripheral compartment.
Metabolism: How is the drug molecule metabolised by the different metabolic enzymes (such as monoamine oxidase or P450 oxidases) in the blood, liver, and other organs and at what rate?
Excretion: How quickly is the drug and its metabolites removed from the body via the kidneys and biliary system?
All of these processes occur simultaneously and at different rates (the different “k” values in the figure below). However, absorption always increases the blood concentration of the drug (since the drug is entering the blood by absorption) and, in the case of IV injection, is irreversible (the drug doesn’t spontaneously burst from the injection site). Metabolism and excretion are also generally irreversible and obviously decrease the blood drug concentration. Distribution, on the other hand, is usually a two-way process, since a drug can both enter and leave the tissues, fat, etc.
Whilst it’s not possible to measure the rates of these processes directly, we can measure the blood drug concentration by taking blood samples at many time intervals following administration. This gives us an overall picture of how these processes together contribute to the drug’s rise and subsequent fall in the blood stream.
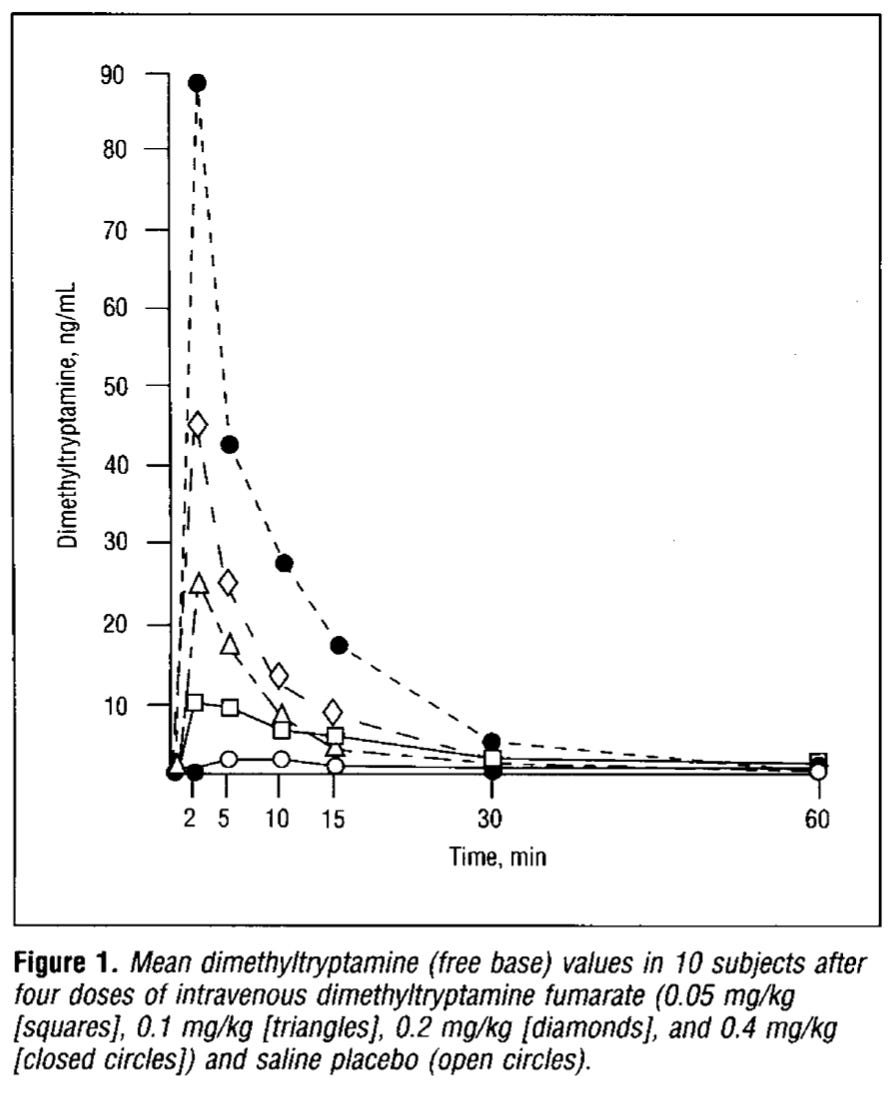
Following a bolus injection (a bolus is a single dose of a drug injected within a short period of time), the blood concentration typically rises very rapidly before reaching a peak and then falling off exponentially as the distribution, metabolism, and excretion begin to dominate. Below are the measured blood DMT concentrations for doses from 0.05-0.4 mg/kg from Rick Strassman’s 1994 paper (link):
The only way to tease apart this blood concentration curve and find the rates of the individual ADME processes is to fit the data to a pharmacokinetic (PK) model using specialised computer software that will try to find a set of rates that best replicates the measured blood concentration-time data. Since distribution to the soft tissues, fats, etc, tends to occur at different rates, fitting of the model often works better if more than one peripheral compartment is used, each with its own IN and OUT rates. Most PK models use either one or two peripheral compartments. Controlling and maintaining a target concentration of anaesthetic drug in the effect compartment (the brain) is the principal aim of the technique known as target-controlled intravenous infusion (TCI), which is used in anaesthesiology to keep a patient unconscious during surgery. DMTx is a variation on this technology that replaces the short-acting anaesthetic with DMT.
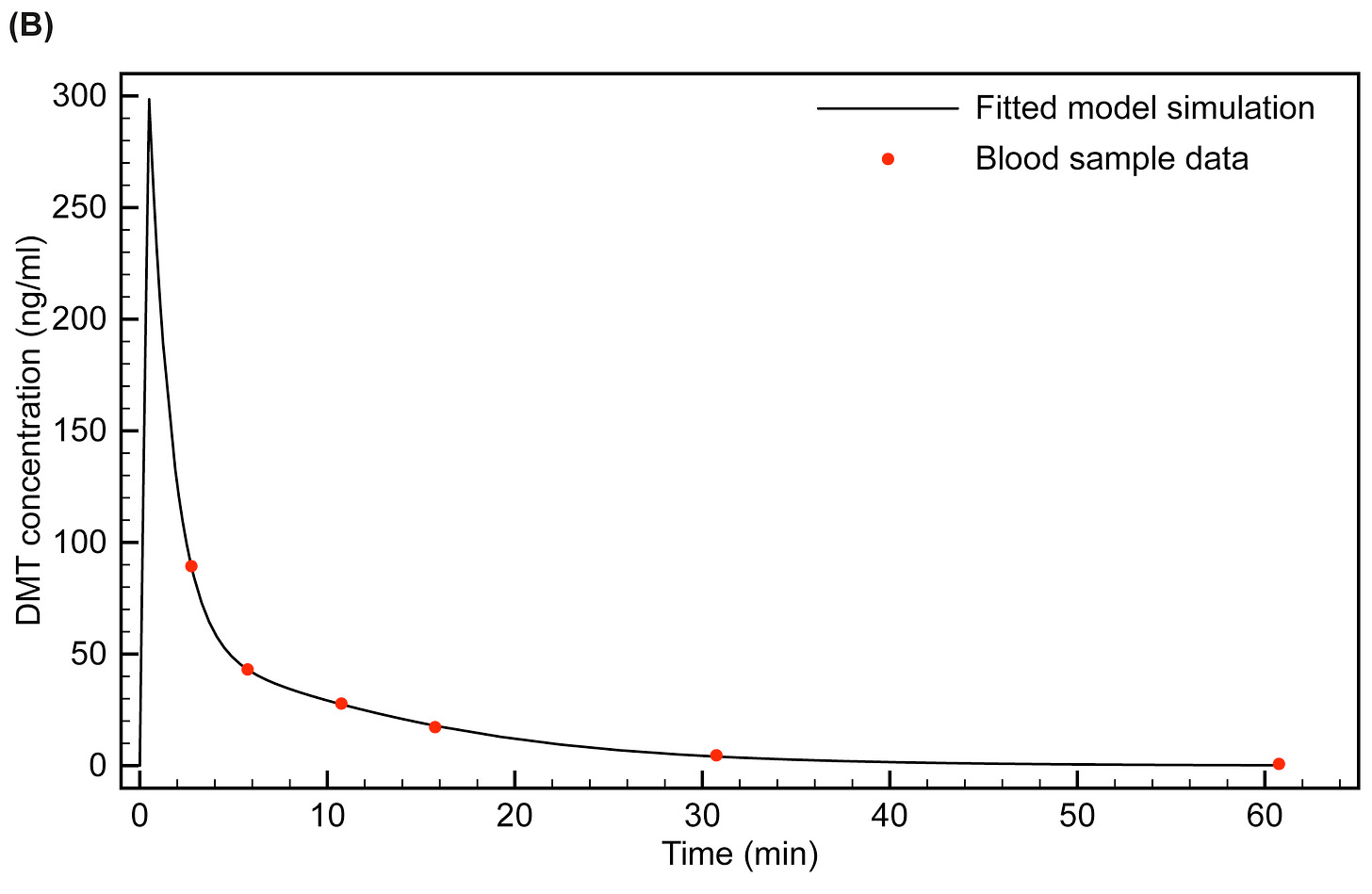
This plot below shows the PK model we fitted to Rick’s blood sample data (at the highest 0.4 mg/kg dose). Note that the actual blood concentration peaks earlier and much higher than at the time the first blood sample was taken.
Our simulations using this model (and based on the known subjective time course of the DMT experience) suggest that “breakthrough” occurs once the brain concentration reaches around 60 ng/ml before peaking at around 100 ng/ml. The subject then exits the DMT space once the brain concentration again drops below 60 ng/ml around 6-7 minutes later.
Once we have an estimate of the ADME rates, we can then manipulate the infusion rate to try and find one that will give us a stable brain drug concentration over time. An efficient way to achieve this is to use the bolus-elimination-transfer (BET) protocol. The basic principal of this protocol is to first use a loading dose (the bolus) to rapidly bring the blood (and brain) drug level to the target concentration. Elimination (by metabolism and excretion) and transfer (distribution to the peripheral compartments) begins immediately and, without further drug administration, the effect compartment (brain) drug concentration will begin to fall exponentially.
However, this rapid elimination-transfer drop off can be offset by beginning a controlled infusion around the time at which the effect compartment drug concentration peaks. So, the idea is to balance the elimination-transfer with the infusion and, assuming the PK model is well-constructed, maintain a stable brain drug concentration for as long as desired. Notice in the simulated plot below how the blood plasma concentration (central compartment) spikes much higher than the brain concentration (effect compartment), since the drug takes time to make its way to the brain and through the blood brain barrier.
We can use the model to simulate any infusion rate we desire to predict how it will affect the brain concentration over time. Below is a simulation of a 2005 study (link) that employed what we thought, upon inspection, seemed like quite a high infusion rate. And, indeed, our simulations suggested that, rather than reaching a sustained plateau, the brain DMT concentration is likely to rise at this infusion rate to almost 150 ng/ml, which we surmised might have been responsible for a number of subjects dropping out of the study owing to overly intense effects. This illustrates the usefulness of simulations in getting the infusion rate right before moving to human studies.
Once a PK model has been developed, it’s also possible to modulate the entry dynamics — the rate at which a breakthrough brain concentration is reached. A slower initial bolus can help to smooth out the rather turbulent rollercoaster phase, which many find unpleasant and disorientating, gradually bringing the brain DMT concentration to the desired level and allowing the subject to settle into the experience. It’s also possible to reach plateaus of experience, stabilising the subject at a lower, sub-breakthrough level for a time, before pushing them further and, perhaps, bringing them back down a level if it becomes a bit too much. Alongside being able to maintain a stable DMT concentration in the brain, this ability to dynamically control the depth of experience in real time is what sets this technology apart from ayahuasca.
Ah, yes, ayahuasca. Every time I discuss DMTx, I can almost guarantee that at least one reader/listener will respond with “Errr, haven’t you heard of ayahuasca?” Although the MAO inhibitors in the ayahuasca brew certainly extend the experience, you’re still tied to the rise and fall of brain DMT levels dictated by its (slowed) pharmacokinetics, and there’s basically no control over the state once the brew has been ingested. That isn’t to say that the DMTx technology is superior to ayahuasca — it’s simply an entirely different way of administering and experiencing DMT. Furthermore, the blood concentration reached with a breakthrough dose of DMT is around 5-6 times higher than achieved with a standard dose of ayahuasca. So, if you want to reach and maintain a breakthrough state with full control, then DMTx is most certainly the better method. And, of course, the purgative side-effects — that is, vomiting and shitting — of ayahuasca are also avoided with DMTx.
If all of this made sense and you’re interested in all the technical details, you can download mine and Rick’s paper in full here :
https://www.frontiersin.org/articles/10.3389/fphar.2016.00211/full











One question that popped into my head was that you are putting in 60-100 ng/ml and after looking it up it says that average levels of serotonin go from 50-200 ng/ml. I have little background in this subject and I’m not sure if it’s even a valid question but does this correlate in anyway since both work on the same 5HT receptors. Or would it have to be that way for anything working in those receptors?
Hi Andrew, love the substack format, reading all your work voraciously. I remember from the Psychadelic Neuroscience videos that you stated neural oscillations were affected and spikes in the theta bandwidth were generally observed towards the end of the trip. Im curious if the same will be true in with infusion technology? Im also extremely curious to hear what your thoughts are on the spike in theta activity....