Anyone with even a passing interest in or unacknowledged dependence upon psychoactive drugs will be familiar with the concept of the “designer drug”: novel drug analogues based on the structure of other, usually more familiar, molecules. If you’re into LSD then you’ll almost certainly be familiar with 1P-LSD and the more adventurous of you might have dabbled in one of PCP’s many alter egos: PCPy (rolicyclidine) or 3-MeO-PCE or, perhaps even, the namesake of the notoriously fragrant antiseptic of my youth, TCP (tenocyclidine).
If it stings, that means it’s working…
Usually, these analogues — around 50 new ones appear on the market each year — are created to sidestep antiquated and entirely counterproductive laws that prohibit various drug molecules based on their structure and often arbitrary classification — a sidestep in a never-ending and ultimately tedious and pointless dance of state anality and intransigence vs human nature. But, fortunately, this isn’t always the case. What was Shulgin if not a pioneer in the construction of designer psychoactive drugs? Shulgin’s peerless work wasn’t aimed at outwitting the US legal system or the DEA (although he certainly had some run-ins in his time), but to create new tools for studying consciousness and for exploring the seemingly limitless reach of the mind into realms only a handful of us hominids are either willing or interesting in reaching.
The action of almost all psychoactive drugs depends upon their interaction with receptors (or sometimes ion channels or pumps) that come factory-equipped with a human brain. Of course, all of these different endogenous receptors have evolved particular roles within our nervous system, but they’re unified in being the means by which molecules outside a neuron can perturb the complex networks of signalling molecules inside that control its function and behaviour. Drug molecules essentially hijack these receptors to manipulate neurons (and thus cortical activity) for their own ends.
The chemical properties of a particular molecule will determine the receptors with which it interacts and its ability to activate those receptors. I like Shulgin’s analogy of the orchestra here (which I might be embellishing a little): Your receptors are like the instruments of an orchestra and, by “playing” particular patterns of receptors, every drug molecule evokes a unique “sound” experienced as the drug effect. For example, whilst DMT and 5-MeO-DMT both depend upon an interaction with the serotonin 5HT2A receptor to elicit their psychedelic effects, they also have different patterns of activity at other serotonin receptor subtypes, which undoubtedly contributes to their markedly different subjective effects.
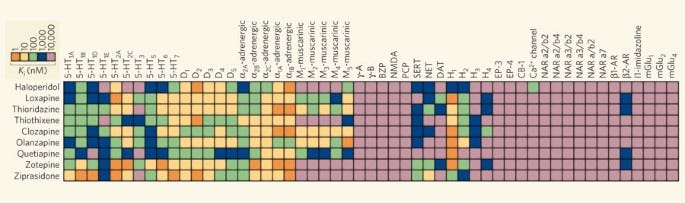
Below shows the binding activity of some commonly used antipsychotic drugs against a range of receptors. Whilst drugs are often marketed as acting primarily at a single type of receptor, they are often active to a greater or lesser degree at many different receptors (this is known as drug promiscuity):
And even this is a somewhat simplified picture, which is complicated further by functional selectivity: there is often more than one way to activate a single receptor subtype. So, the overall effect of a drug depends not only on the pattern of different receptors activated, but how each receptor is activated (you can imagine this kind of like the particular timbre a musician evokes from their instrument).
Much of the difficulty in trying to predict the subjective effects of a drug molecule lies in this complexity that emerges from the particular pattern of activation of all of these different receptors. This is why Sasha Shulgin focused on making large numbers of new molecules and testing them on his own brain (and the brains of his “research group” members) rather than attempting to design a molecule with particular subjective effects. Whilst it’s often possible to predict (often incorrectly) using receptor binding or computational molecular docking studies whether or not a molecule is likely to be broadly psychedelic, it remains practically impossible to predict its unique nuanced subjective effects, such is the promiscuity of most drug molecules at different receptors. And such is the complexity of the human brain!
So, if we can use novel designer molecules to generate a particular subjective “sound”, might it be possible, rather than finding new ways to play the instruments already present, to add entirely new instruments to the orchestra? In other words, rather than designing a drug molecule to fit endogenous receptors, can we design receptors — and insert them into a human brain — to fit a drug molecule? Well, it turns out that we can and, indeed, these designer receptors already exist (although not yet for humans!)
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), as their name implies, are receptors engineered to be activated only by otherwise inert exogenous molecules, but unresponsive to endogenous molecules. So, unlike your factory-equipped receptors, which are always being stimulated to a greater or lesser extent by their endogenous ligands (such as serotonin or dopamine, etc), DREADDs will remain silent until the exogenous molecule is administered.
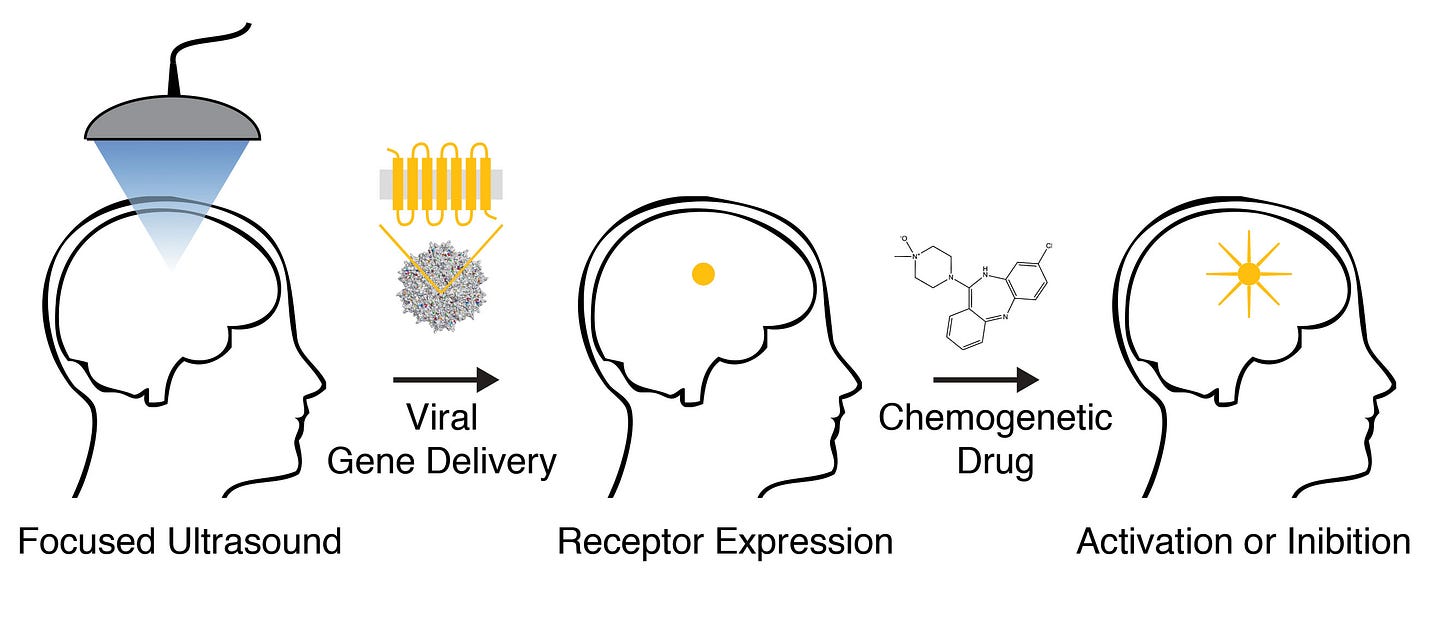
To make a DREADD, you first need the gene sequence of a standard receptor with the desired signalling properties, such as a 5HT2A receptor coupled to the G-protein and arrestin pathways. Next, you need to generate a series of mutated receptor genes (i.e. genes with a slightly altered DNA sequence), which are used to express the mutant receptor proteins themselves. So, you now have a series of receptors with slightly different structures and, often, slightly different ligand binding sites (where the drug molecule binds!). It’s then a relatively simple task to screen these mutant receptors against biologically inert synthetic molecules and see which ones stick. Once a candidate receptor that binds to the molecule has been identified, the mutant gene is implanted into a virus programmed to insert the receptor DNA into a specific neuron type. Precisely localised ultrasound can also be used to temporarily open out the normally extremely tight pores in the blood brain barrier in a specific region of the brain, allowing the virus sneak through the gap and reach the desired neurons. The hope is that these neurons will produce the mutant receptor from the mutant gene and that it will be inserted into the neuronal membrane, alongside the endogenous receptors. This mutant receptor will sit quietly and patiently until you administer your biologically inactive synthetic molecule, which will bind and activate the mutant receptor exclusively.
For example, the DREADD known as hM4Di is a mutated form of the muscarinic M4 receptor which, rather than binding to acetylcholine, binds only to an otherwise pharmacologically inert molecule, clozapine-N-oxide (CNO), but retains the same intracellular signalling properties — a general inhibitory effect on neurons — as the natural receptor. Bryan Roth’s group selectively expressed hM4Di in hippocampal neurons (a part of the cortex important for memory formation) of rats and used it to specifically inhibit activity in this part of the brain, disrupting their ability to form new memories (LINK).
Using these DREADDS, receptors with specific signalling properties — coupled to Gs, Gi, and other G-proteins, for example — can be designed and inserted into specific cell types, such as cortical pyramidal cells, to control their activity in a highly selective manner using molecules that have no effect on other receptors or neurons. Different DREADDS can be embedded in different neurons to create an entirely novel “orchestra” of receptors that can be activated to varying degrees, each modulating its own neuron type dependent on the controlled administration of its own specific activator molecule. A cocktail of activator molecules could be administered to elicit a precisely controlled pattern of receptor, and thus neuron, activation so as to generate a desired psychoactive effect. Naturally, we’d first need to find some human subjects willing to undergo the receptor implant procedure but, based on my emails, I don’t think we’d be short of volunteers.
Of course, even with such a sophisticated neuropharmacological technology, the problems inherent in relying upon exogenous molecules remain. Even if the drug is delivered by intravenous injection, bypassing issues with gastrointestinal tract absorption and first-pass metabolism by the liver, the same dose might well have different effects on different people, depending upon idiosyncratic variables controlling absorption into the brain, metabolic enzyme expression, and so on. Ideally, we ought to think towards dispensing with exogenous molecules entirely and rely upon more rapid, less invasive, and more precisely controllable means of activating our implanted receptor orchestra.
Magnetoreceptors are a relatively new — at least to science — class of receptors activated not by the binding of ligands but by the application of a magnetic field. Rather than a ligand binding site, the receptor is coupled to superparamagnetic nanobeads about the same size as a synaptic vesicle. Upon exposure to the magnetic field, the nanobeads pull the receptors into a cluster which activates them (LINK). Engineered magneto-forms of ion channels have already been used to control the behaviour of both zebrafish and mice (LINK).
Since magnetic fields are transparent to biological tissue, including bone, activation of the receptors is rapid, safe, and can be controlled remotely. Once a brave volunteer has been equipped with this designer receptor orchestra using viral transfer, they would lie down in a comfortable pod and be fitted with a helmet within which the electromagnets are arranged around the skull. Initiating the psychedelic experience — perhaps a day trip to the DMT Cosmic Circus — would be as simple as flicking the switch and giving a wink and a wave…
See you sooooooooooooooon……











Could the insertion of designer receptors at specific brain sites one day potentially aid in permanently restoring proper neuronal network connectivity in certain diseases and disorders?
Sincerely thankful for all your public content, it's greatly enhancing my pharmacology studies.
Why hasn’t molecular bio post 93 ie SNPs and genotypical receptor selectivity and counter consequence not been mentioned yet? Or is that after the interest-popularity wave?