Psychedelic Amphetamines, Speedy Amphetamines, Entactogenic Amphetamines...
The neuropharmacology of the substituted amphetamines.
One of the most fascinating aspects of neuropharmacology, for me at least, is how closely related molecules, perhaps differing only by the presence or absence of a methyl group (think DMT vs 5-MeO-DMT), can have dramatically different effects on the brain and consciousness. Whilst we understand much about the way molecules interact with receptors, how this modulates neuronal behaviour and, ultimately, how this affects both global and regional brain activity, there’s still so much we don’t understand.
Trying to work out why a particular molecule has a particular psychological effect is challenging, but trying to predict the psychoactivity profile of a drug before it’s put into that massively complex system that is the human brain is often well beyond our abilities. There’s a reason Alexander Shulgin assayed all of his new creations on his own brain — there’s just no better way to do it. However, with modern pharmacological techniques, we can at least make a rough prediction as to a molecule’s likely psychoactive effects by measuring how it interacts with various receptors and transporter proteins.
In the last post I discussed Shulgin’s eleven essential amphetamines: a group of psychoactive molecules based on the amphetamine skeleton, but with varying groups on the phenyl ring.
Beyond these, dozens of substituted amphetamines have been developed, mainly for clinical and basic research purposes, but also as recreational drugs. Whilst their effects vary widely and often unpredictably, we can use their measured interactions between receptors and protein transporters to both predict and explain the effects of a particular type of amphetamine. We’ll start with the simplest, amphetamine itself, and the most famous of the substituted amphetamines, MDMA.
Amphetamine is the prototypic stimulant, causing psychostimulation, euphoria, and, with repeated use, dependence. MDMA, on the other hand, is the prototypic entactogen (the original term empathogen has fallen out of favour). The psychostimulant effects of MDMA are much less prominent, and there’s little propensity for physical dependence, but there’s a strong feeling of well-being, empathy, and general “prosociality” after consuming the drug (hence MDMA and drugs like it often being referred to as “love drugs”). With some users, generally at higher doses, mild psychedelic effects can also be experienced. The other substituted amphetamines and related drugs usually fall somewhere between “amphetamine-like” and “MDMA-like’, with psychedelic effects adding another dimension to the experience with certain molecules.
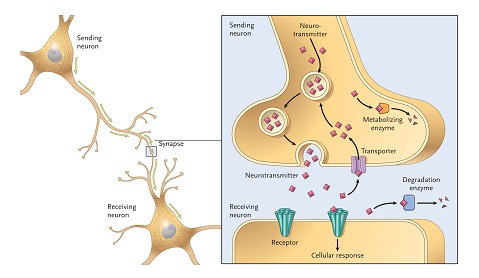
Understanding why certain amphetamines are speedy, others more calm and entactogenic, and others decidedly psychedelic, requires us to think about how and why they modulate certain endogenous neuromodulator systems in the brain. Neuromodulators are a class of neurotransmitter that regulate the activity and behaviour of neurons across the brain. The three most important in the action of the substituted amphetamines are known collectively as the monoamines: dopamine, norepinephrine (aka noradrenaline), and serotonin.
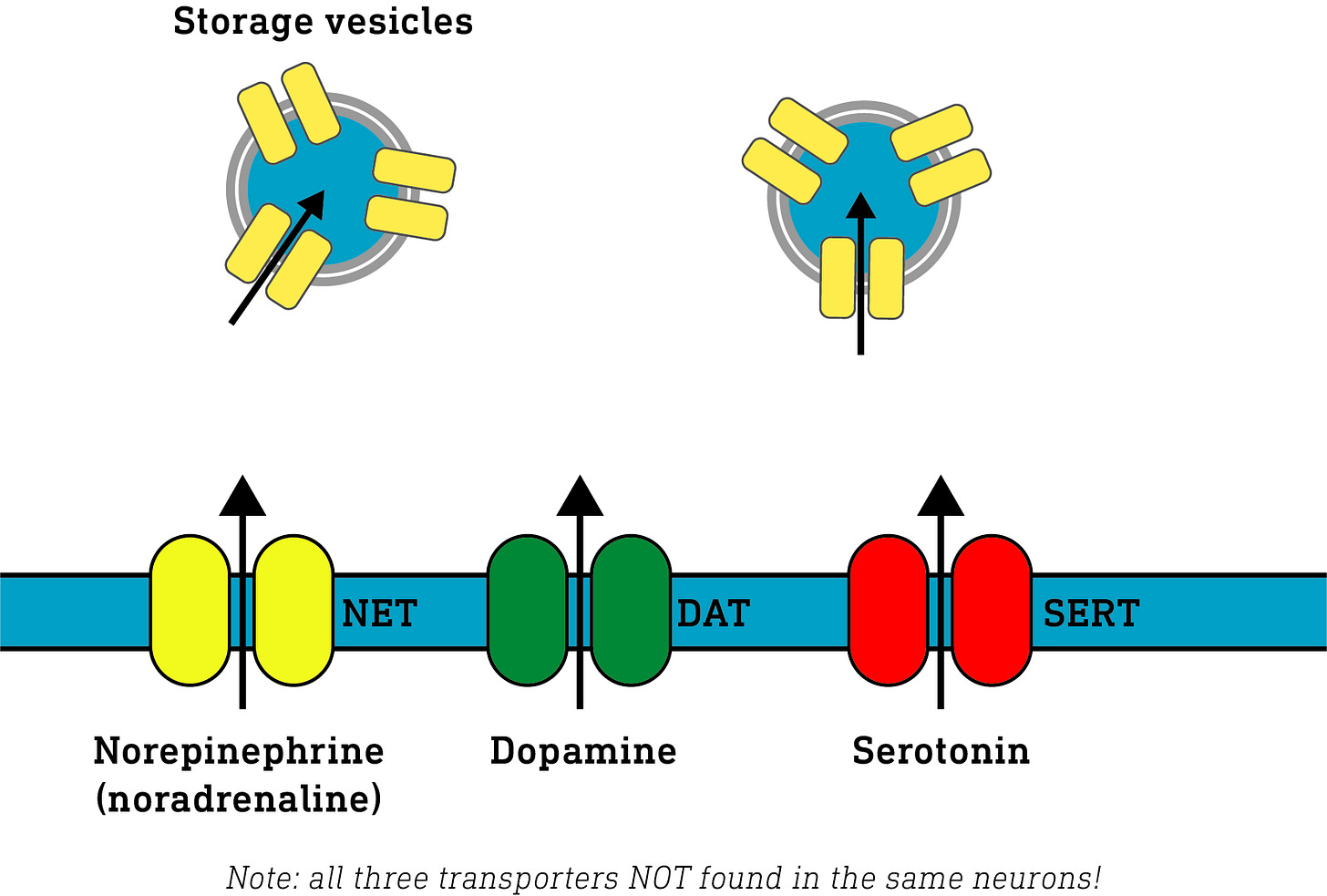
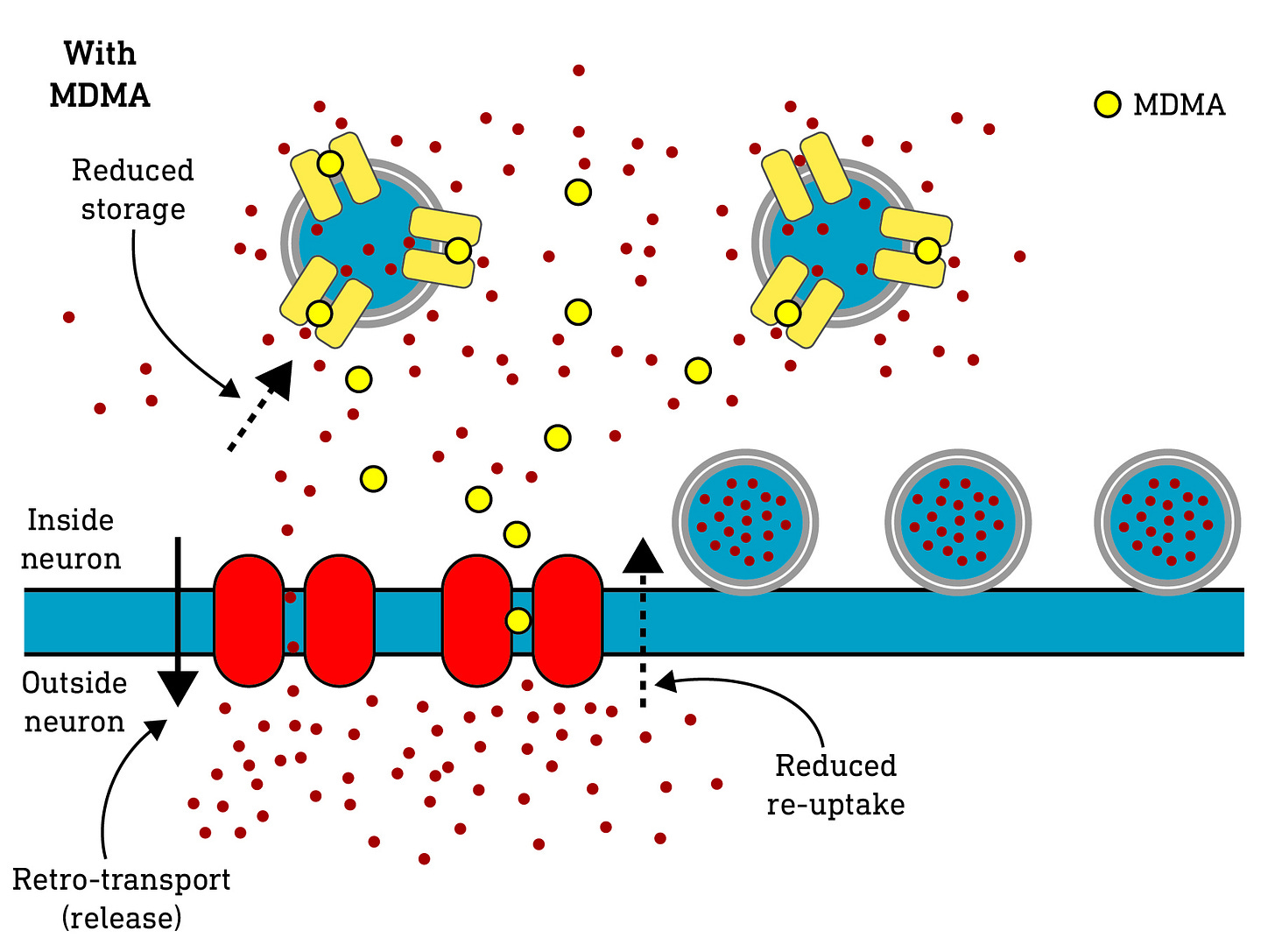
These are released by specialist neurons (i.e. each neuron type can only release one type of neuromodulator) and modulate the activity of other neurons that bear receptors for those neuromodulators. To limit the amount of time these neuromodulators are active, they are either broken down or taken back up into the neuron that released them using specialist monoamine transporter proteins. Once back inside the neuron, they can either be re-packaged for release again or stowed away in storage vesicles for later use. Dopamine, norepinephrine, and serotonin utilise different re-uptake transporters (DAT, NET, and SERT, respectively)
The substituted amphetamines can interact with these monoamine transporter systems and broadly exhibit two related effects:
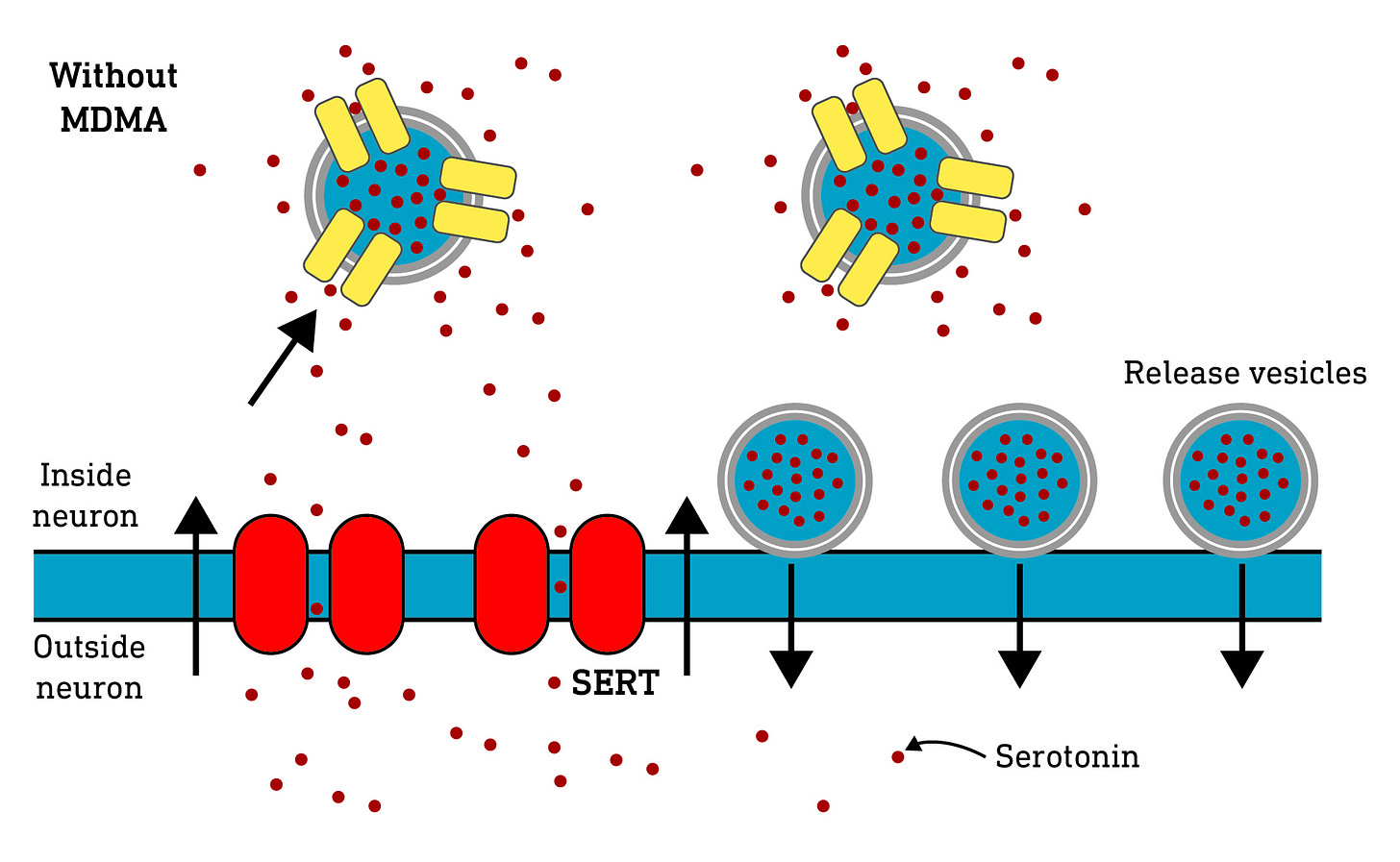
They can block the re-uptake proteins and prevent the monoamine being transported back into the neuron, increasing the time it spends outside the neuron modulating the other neurons. Molecules that do this are known as re-uptake inhibitors.
They can “hijack” the re-uptake transporter and be taken up into the neurons themselves. As they build up, they begin to displace the endogenous monoamine neuromodulators, as well as interfering with the protein transporters that package the monoamines away for storage. This causes free (i.e. not stored away) monoamines to build up inside the neuron and, as a consequence, they begin to spill out of the re-uptake transporters. In other words, they cause the the re-uptake transporters to go into reverse, pumping monoamines out of the neuron where they can modulate other neurons. Molecules that do this are known as monoamine releasers.
Note that most substituted amphetamines work both as re-uptake inhibitors and releasers.
The overall effect of both mechanisms is to increase the amount of monoamine outside the neuron and thus increase its effects. However, not all amphetamines exhibit effects on all three monoamine systems equally, and this is largely the basis for their differential effects when consumed (ignoring psychedelic effects for the time being). Whether or not a particular substituted amphetamine will interact with a particular re-uptake transporter will depend upon its molecular structure. This is the same principle that explains why certain psychedelic drugs bind to only certain serotonin receptor subtypes.
Without going deep into the neuroscience, increasing the concentration of each of the monoamines outside neurons will have distinct psychological effects:
Dopamine: Euphoria, increased motivation, pleasure, and satisfaction. Dopamine is an important component of the brain’s reward circuitry that regulates the motivation to seek things important to survival and reproduction — food and sex, for example. Drugs that enhance dopamine transmission often have the potential for physical dependence (i.e. addiction).
Norepinephrine: Psychostimulation — increased arousal, alertness, mental energy, and focus. This is the basic “speedy” effect of some drugs.
Serotonin: Often referred to as the brain’s “feel good chemical”, when serotonin levels are elevated, you feel happier, calmer, more sociable. This is the “entactogenic” or “empathogenic” effect.
Of course, this is far from the whole picture, and monoamines have many more roles both inside and outside the brain. However, this limited overview is useful in understanding and explaining the psychological effects of the different substituted amphetamines.
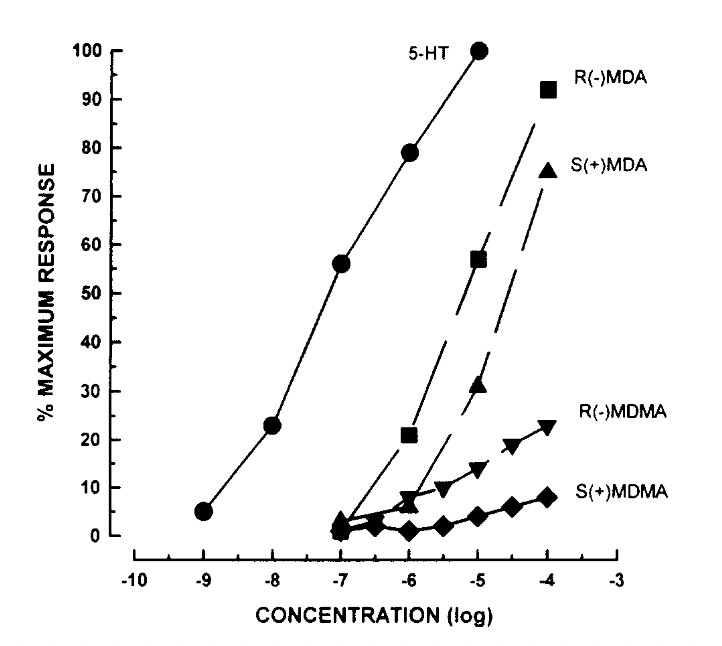
Let’s start with the prototypic stimulant — amphetamine itself. This simplest of the amphetamines interacts preferentially with the DAT and NET transporters and, as such, causes an increase in both dopamine and norepinephrine signalling. This elicits the mixture of psychostimulation and euphoria characteristic of this drug. MDMA, on the other hand, has much weaker effects on both DAT and NET, but preferentially interacts with the SERT transporter and thus elevates serotonin release, causing its characteristic entactogenic and pro-social effects. Note that its low DAT (dopamine) activity also means it has a much lower propensity to cause dependence than amphetamine.
Such is the stark contrast between amphetamine and MDMA on the DAT and SERT transporters, the ratio of DAT to SERT activity of a particular molecule can be used to predict whether it will be more “amphetamine-like” or “MDMA-like”. The ability of a molecule to interfere with particular re-uptake transport mechanisms is relatively easy to measure in the laboratory, so this data is often readily available in the academic literature.
A high DAT/SERT ratio, meaning the molecule elevates dopamine much more than serotonin, suggests the drug will be more amphetamine-like.
A low DAT/SERT ratio suggests the opposite, that the drug is likely to be more similar to MDMA.
A ratio of 1 will indicate equal activity at both DAT and SERT transporters.
Amphetamine itself has a DAT/SERT ratio of over 10, whereas MDMA’s DAT/SERT ratio is 0.08. Para-methoxy-amphetamine (PMA), discussed in the last post, has a DAT/SERT ratio of 0.03, suggesting its effects will be very close to MDMA, and indeed this is what’s generally reported and is why PMA can be substituted for and mis-sold as MDMA. The same applies to lesser-known substituted amphetamines, such as 4-MA, MBDB, and MDEA, which all have similar DAT/SERT ratios to MDMA and comparable effects.
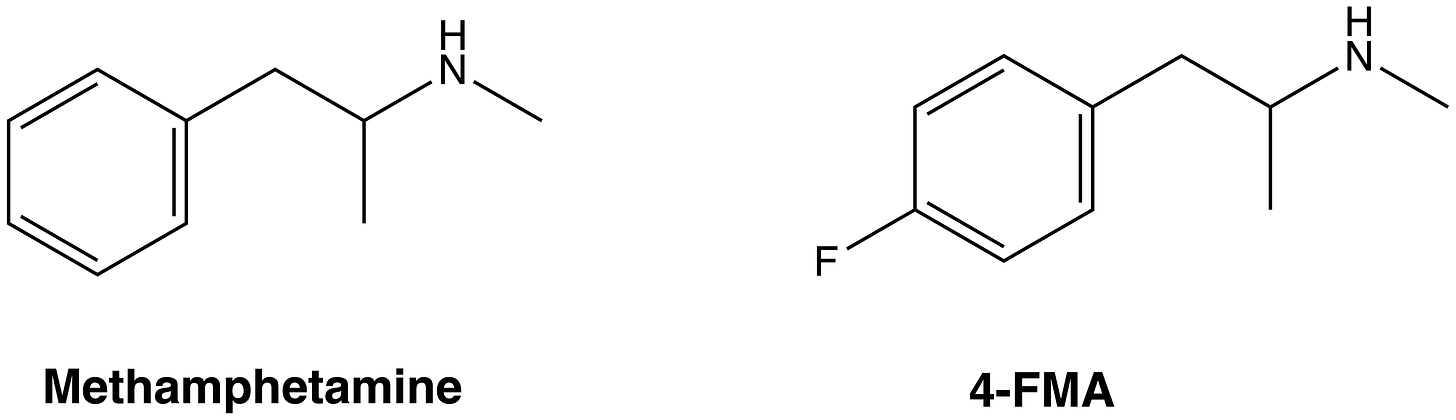
Methamphetamine, just like amphetamine, has a DAT/SERT ratio of >10, but is an even more potent norepinephrine re-uptake inhibitor/releaser (making it more psychostimulatory than amphetamine). However, the closely-related 4-FMA (4-fluoromethamphetamine), differing only by a fluorine atom at the 4-position on the phenyl ring, has a DAT/SERT ratio of 1.1, meaning it increases both dopamine and serotonin about equally, as well as having activity at NET. So, we should expect amphetamine-like euphoric and psychostimulatory effects and MDMA-like entactogenic effects from 4-FMA. And, indeed, this is borne out by subjective reports.
Whilst the DAT/SERT ratio is useful in identifying molecules likely to be either amphetamine-like or MDMA-like, it doesn’t take into account activity at the NET transporter, which will determine its level of psychostimulation or “speediness”. MDMA itself has moderate potency as a NET re-uptake inhibitor and norepinephrine releaser, adding a psychostimulation dimension to the subjective experience. If you want maintain the MDMA-like effects whilst ramping up the speediness, then perhaps 5-APB (5-(2-aminopropyl)benzofuran) will do the trick. 5-APB is a member of the benzofuran class of subsituted amphetamines. Its DAT/SERT ratio is similar to that of MDMA, but is also a more potent inhibitor of the NET transporter (and a norepinephrine releaser), which explains why it’s often described as a more stimulating alternative to MDMA.
On the other hand, if you want to remove the speediness aspect entirely from the MDMA experience, the you should look for a molecule with a low DAT/SERT ratio but with very low activity at the NET transporter. In that case, MMAI (5-methoxy-6-methyl-2-aminoindane) might be what you’re looking for: Its DAT/SERT ratio is much lower than that of MDMA (meaning it’s a highly selective serotonin releaser with no significant effect at DAT), but has almost no effect on NET.
Many substituted amphetamines also have a pronounced psychedelic component to their subjective effects, which is not accounted for by their activity at the monoamine re-uptake transporters, so we need to look for additional receptor activity to explain this. The most obvious explanation would be activity at the serotonin 5HT-2A receptor, which is the primary locus of action of the classic psychedelics, such as LSD and psilocin. Although it would be a stretch to call MDMA a psychedelic, some level of psychedelic effect is reported by many users. MDMA has a relatively low potency as an activator of the 5HT2A receptor, but it’s plausible that this might account for the mild psychedelic effects. MDA, however, is around ten times more potent at the 5HT2A receptor and is often described as being more psychedelic than MDMA. It also happens to be an active metabolite of MDMA, meaning it’s generated inside the body after ingestion. As such, it’s reasonable to surmise that MDA might be responsible for the reported psychedelicity of MDMA, particularly towards the tail end of the experience, rather than MDMA itself.
The rather rare 5-IAI (5-iodo-2-aminoindane), originally developed by Dave Nichols’ group at Purdue, has a low DAT/SERT ratio, predicting MDMA-like activity, whilst also being a highly potent 5HT2A activator, suggesting it might be even more psychedelic than MDA. However, reports of the effects in humans are scarce and mixed.
Of course, the brain is an extremely complex system and we can never predict with absolute certainty the psychological effects of a particular drug without actually ingesting it: Interactions with other receptors and transport systems not considered here can have modulatory effects on the subjective experience, as can a molecule’s particular pattern of metabolism, distribution, and excretion. I never said neuropharmacology was simple.
Plotting the DAT/SERT ration against activity against the NET transporter, we get a nice visual impression of the relative effects of these substituted amphetamines:














Sir, are you aware of the old obscure stimulant 4-methylaminorex? It’s one that many in to the psychostimulants, after hearing testimonials of some who have had this rare chemical say it’s the holy grail of psychostimulants, with some preferring it’s effects over even methamphetamine. And what’s interesting is that this drug appears to be a unique orphan drug, seeming not at all related to the amphetamine and even cocaine class.
This was excellent. Do you happen to know the affinities of the molecules at TAAR1?